Treatment of Infantile Neuroaxonal Dystrophy (INAD) with a Stabilized Polyunsaturated Fatty Acid Drug: Report of 2 cases
Atlantic Health Objective:
Lipid peroxidation (LPO) accumulation is present in INAD. RT001 prevents LPO and
reverses its effects in nonclinical models of INAD. To evaluate RT001 in INAD, we
observed the clinical course of two subjects who received RT001 for a minimum of 6
months.
INAD Background:
INAD is an ultra-rare (1:1,000,000), autosomal recessive, neurological disorder. Disease
onset may start at 6 months of age with slowing of motor and cognitive development
and regression of previously acquired skills. Death usually occurs between the ages of
5 to 10 years, often from loss of bulbar function leading to aspirational pneumonia.
The genetic basis of INAD is variation in the PLA2G6 gene (chromosome 22q13.1)
which encodes an 85 kDa group IV calcium independent phospholipase A2β (iPLA2β).
The enzyme is responsible for the selective hydrolysis of the sn-2 ester bond of
glycerophospholipids to release free polyunsaturated fatty acids (PUFAs). This is a
critical housekeeping function in all cells membranes but in particular those exposed to
high oxidative stress such as mitochondrial membranes in high energy tissues.
Design/Methods:
Two subjects with classical INAD signs and 2 mutant PLA2G6 alleles were enrolled in
Expanded Access Protocols. Subjects were scored with a novel rating scale of
development in INAD, consisting of 32 elements. Subjects received 1.8 g of RT001
twice daily. The INAD Scale was repeated every 6 months.
Results:
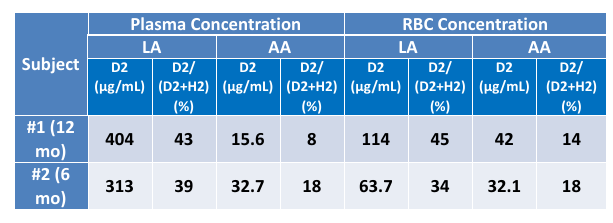
• Pharmacokinetic analysis
Significant plasma levels of deuterated linoleic acid (D2LA) and arachidonic acid
(D2AA) were seen relative to the non-deuterated analytes (H2LA, and H2AA) for
both subjects. Membrane incorporation was demonstrated in the RBC
concentrations:
• Efficacy analysis
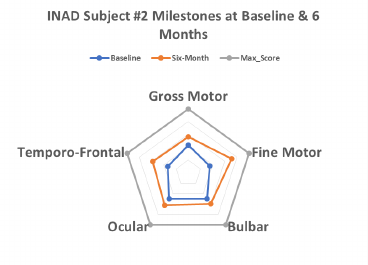
Both subjects have shown improvement over baseline for INAD rating score
(Subject 1: 7 to 26; Subject 2: 27-39; max 62), and in the number of elements
showing improvement (Subject 1: 16; Subject 2: 12; max 31). Both subjects
showed improvement in at least one component of each developmental category.
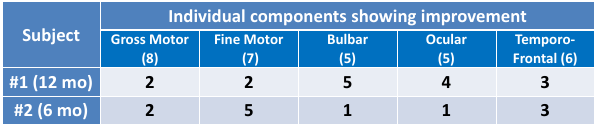
Efficacy Analysis (cont.)—Results of INAD Rating Scale
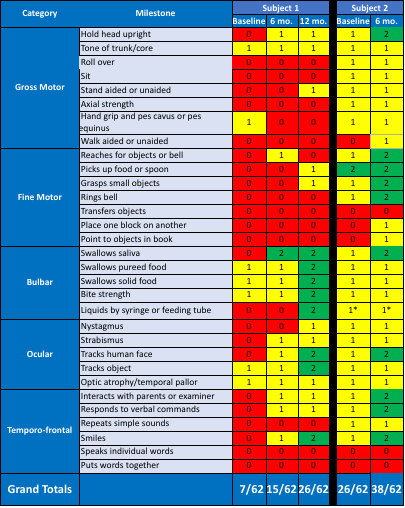
red (0) for severely impaired, yellow (+1) for moderately impaired, and green (+2) for mildly impaired or no impairment
- Subject receives liquids by syringe as a convenience
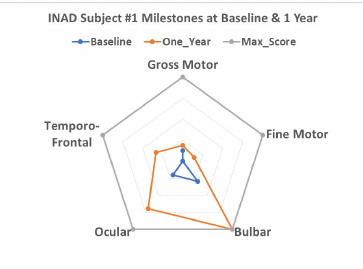
Efficacy Analysis (cont.)—Radar plots for INAD Rating Scale
• Safety Analysis
- RT001 has been administered as an additive to food
without difficulty - No drug-related adverse events have occurred.
Conclusions:
- Administration of RT001 orally to 2 subjects with INAD was
accompanied by significant plasma levels of D-linoleic
acid, and D-arachidonic acid, with incorporation of both
compounds into RBC membranes. - Within one month of dosing and continuing through 1 year
and 6 months respectively, both subjects with INAD have
demonstrated arrest of disease progression, with return of
some lost developmental milestones. - In a disease marked by inexorable decline and milestone
loss, stabilization of regression and recovery of lost
milestones may indicate some level of beneficial effect
from RT001 in INAD and warrants further study.
